Autoradiography and Recent Paintings by Michael Walker
My most recent series of larger paintings are painted in oil and alkyd resin on canvas, mounted on panel. Buried in these paintings, there are three 14″ x 17″ monochrome acrylic images that are sandwiched between the layers of gesso primer. These images are hidden from the naked eye beneath the final painted image. The first image is painted in Burnt Umber acrylic paint due to its high manganese content. On top of the brown image and more gesso layers, a second image is painted in pure Ivory Black, due to its high phosphorus content. On top of the black image and more gesso layers, a third image is painted in pure Cobalt Blue due to its high cobalt content. These images are intentionally layered and calculated to show up in a future autoradiograph as three separate images.
In order to fully engage with these paintings, they will need to be autoradiographed to expose the additional images that provide added context to the work. These images become ephemeral radiation that emerges, then retreats from view and sometimes later reappears in different contexts. An autoradiograph is a noninvasive imaging process used by contemporary art conservators to examine the underlying layers of paint hidden from the naked eye. Neutron induced autoradiographs are created by using a linear particle accelerator or a research nuclear reactor. To produce an autoradiograph, a painting is placed in a beam of radioactive thermal neutrons and then a series of x-ray films (14″ x 17″ inches in size) is placed on the painting surface at different time intervals to be exposed by the different irradiated pigments. If timed properly, each developed film can show a specific pigment that contains a specific radioactive element. For example, Manganese, the main element in Burnt Umber, will show up on an exposed film at approximately 2 hours after irradiation. Approximately 2-10 days later, phosphorus, the main element in Ivory Black, will show up on an exposed film revealing the second image. After 30+ days, the cobalt isotope shows up on an exposed film, revealing the third image.
Below, are my research results and tests conducted at the Royal Military College Kingston, Ontario Canada, June-August 2004 with supervisor Dr. Leslie G. I. Bennett Department of Chemistry and Chemical Engineering Royal Military College Kingston.

Research Test Films From Orange Painting (Oil)
Tests Conducted at Royal Military College Kingston, Ontario Canada, June-August 2004
Supervisor: Dr. Leslie G. I. Bennett
Department of Chemistry and Chemical Engineering
Royal Military College Kingston, Ontario 2004
These are the film results from a 5 by 7 inch sample painting of an orange radiated using the research nuclear reactor at RMC in Kingston. Underneath the orange, two burnt umber bars (manganese), an French ultramarine blue(sodium) star and a cadmium red (cadmium) circle where painted.
Film exposure information is as follows:
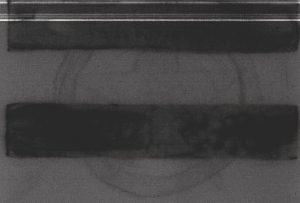
Figure 1: 15 Minutes to 1 Hour After Irradiation
This paint was irradiated for 30 seconds on July 22, 2004 from 9:37:00 to 9:37:30.This film was placed in contact with the sample at 9:52 July 22, 2004 and removed at 10:52 on July 22, 2004. Here we see the two bars of manganese burnt umber showing clearly.
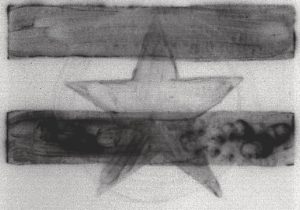
Figure 2: 5 Hours to 1 Day After Irradiation
This paint was irradiated for 30 seconds on July 22, 2004 from 9:37:00 to 9:37:30. This film placed in contact with the sample at 14:18 July 22, 2004 and removed at 9:37 on July 23, 2004.
At this point we see still see the two bars and now the French ultramarine star is starting to appear.
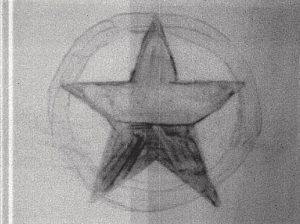
Figure 3: 1 Day to 4 Days After Irradiation
This paint was irradiated for 30 seconds on July 22, 2004 from 9:37:00 to 9:37:30. This film was placed in contact with the sample at 9:38 July 23, 2004 and removed at 9:52 on July 26, 2004
At this point, the bars have disappeared, and the star has revealed itself as two thicknesses of paint. The top half application of ultramarine was thicker than the bottom half which shows darker due to the higher concentration of pigment. Also the cadmium circle is beginning to show.

Figure 4: 4 Days to 6 Days After Irradiation
This paint was irradiated for 30 seconds on July 22, 2004 from 9:37:00 to 9:37:30. This film was placed in contact with the sample at 9:53 July 26, 2004 and removed at 12:30 on July 28, 2004.
At this point in the testing times, there is only faint showing of the star and circle. The cadmium image would show best after day six, between 8-12 days.
Research Test Films From Individual Oil Paint Colours
These are the film results from testing the oil paints. Twenty paints were tested under with two irradiation times. The first six films are for an irradiation time of 30 seconds and the next five films are from a 15-minute irradiation. The paints tested are as follows:
Paint Number | Paint Type |
| 1 | Titanium White |
| 2 | Cobalt Blue |
| 3 | Ivory Black |
| 4 | Ultramarine Blue |
| 5 | Burnt Umber |
| 6 | Cadmium-Barium Yellow (Light) |
| 7 | Cadmium Yellow (Light) |
| 8 | Yellow Ochre |
| 9 | Burnt Sienna |
| 10 | Raw Sienna |
| 11 | Raw Umber |
| 12 | Cadmium Red |
| 13 | Sap Green |
| 14 | Dioxazine Purple |
| 15 | Alizarin Crimson |
| 16 | Zinc White |
| 17 | Pthalo Blue |
| 18 | Scarlet Lake |
| 19 | Lead White |
| 20 | Viridian |
Films are as follows:
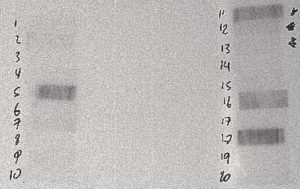
Figure 1: 20 to 30 Minutes After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 11:08 on June 24, 2004 and removed at 11:13 on June 24, 2004
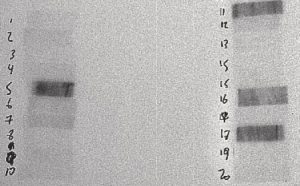
Figure 2: 30 to 45 Minutes After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 11:15 on June 24, 2004 and removed at 11:28 on June 24, 2004
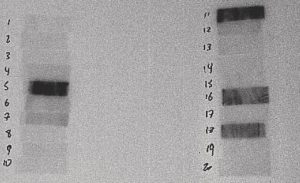
Figure 3: 45 Minutes to 1 Hour After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 11:30 on June 24, 2004 and removed at 11:58 on June 24, 2004
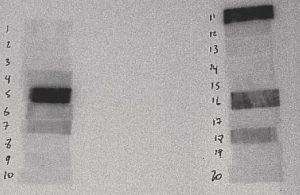
Figure 4: 1 to 2 Hours After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 12:00 on June 24, 2004 and removed at 12:57 on June 24, 2004
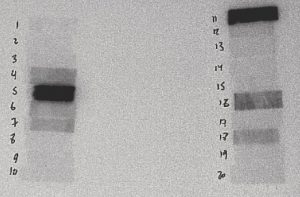
Figure 5: 2 to 3 Hours After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 12:59 on June 24, 2004 and removed at 14:50 on June 24, 2004
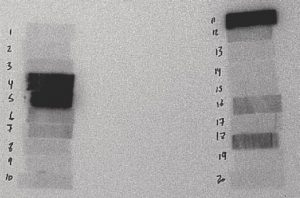
Figure 6: 3 Hours to 1 Day After Irradiation
The film above is from a 30-second irradiation on June 24, 2004 from 10:48:07 to 10:48:37. This film was placed in contact with the sample at 14:51 on June 24, 2004 and removed at 12:41 on June 25, 2004
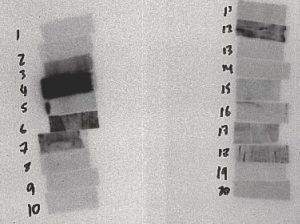
Figure 7: 2 to 3 Days After
The film above is from a 15-minute irradiation on July 6, 2004 from 9:24:33 to 9:42:33. This film was placed in contact with the sample at 13:02 on July 8, 2004 and removed at 12:13 on July 9, 2004
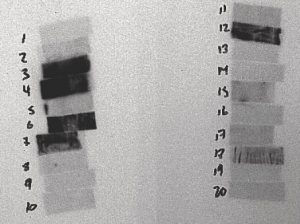
Figure 8: 3 to 6 Day After Irradiation
The film above is from a 15-minute irradiation on July 6, 2004 from 9:24:33 to 9:42:33. This film was placed in contact with the sample at 12:16 on July 9, 2004 and removed at 13:40 on July 12, 2004

Figure 9: 6 to 8 Days After Irradiation
The film above is from a 15-minute irradiation on July 6, 2004 from 9:24:33 to 9:42:33. This film was placed in contact with the sample at 13:42 on July 12, 2004 and removed at 8:51 on July 14, 2004

Figure 10: 8 to 13 Days After Irradiation
The film above is from a 15-minute irradiation on July 6, 2004 from 9:24:33 to 9:42:33. This film was placed in contact with the sample at 8:53 on July 14, 2004 and removed at 14:25 on July 14, 2004

Figure 11: 13 to 18 Days After Irradiation
The film above is from a 15-minute irradiation on July 6, 2004 from 9:24:33 to 9:42:33. This film was placed in contact with the sample at 14:30 on July 19, 2004 and removed at 9:52 on July 23, 2004the
